| ||||
What Is a Lipid Molecule?
Organic Chemistry of Fats, Phospholipids,
Waxes & Steroids
Organic molecules are compounds that contain carbon-hydrogen bonds and are found in living things.The major classes of organic molecule are carbohydrates, proteins, lipids and nucleic acids.
Article Summary: What are the different kinds of lipid molecules? What is the difference between saturated and unsaturated fats? Read on and discover.
Organic Chemistry: What Is a Lipid Molecule?
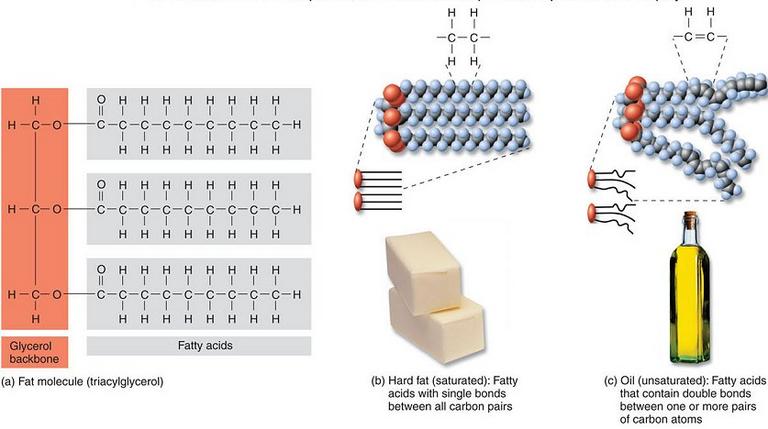
Unsaturated fats (oils):These fats are mostly derived from plant sources. There are double bonds between some of the carbons in the hydrocarbon tails, causing the tails to bend or “kink.” These kinks in the hydrocarbon tails prevent the tails from packing closely together. This is why unsaturated fats are liquid at room temperature.
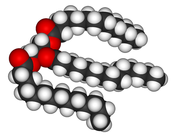
Fats
Fats and oils are made from two kinds of molecules:
- glycerol (a type of alcohol)
- three fatty acids (so known as triglycerides)
Saturated fats: These types of fat are mostly derived from animal sources. There are single bonds between the carbons of their fatty acid tails, meaning that each carbon is bonded to maximum number of hydrogens possible. Therefore the hydrocarbon chains in these fatty acids are fairly straight and packed closely together. This is why saturated fats are solid at room temperature.
You have free access to a large collection of materials used in a college-level introductory Cell Biology Course. The Virtual Cell Biology Classroom provides a wide range of free educational resources including Power Point Lectures, Study Guides, Review Questions and Practice Test Questions.
 | ||||||
SPO VIRTUAL CLASSROOMS
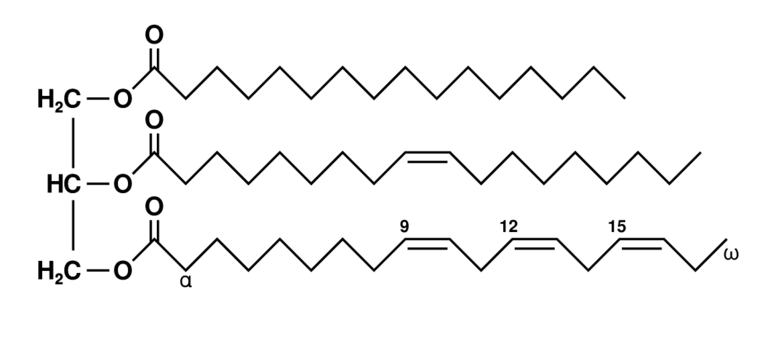
Unsaturated fat triglyceride. Left part: glycerol, right part from top to bottom: palmitic acid, oleic acid, alpha-linolenic acid.
The SPO website is best viewed in Microsoft Explorer,
Google Chrome or Apple Safari.
Page last updated: 8/2015
 | ||||||
SPO is a FREE science education website. Donations are key in helping us provide this resource with fewer ads.
Please help!
(This donation link uses PayPal on a secure connection.)
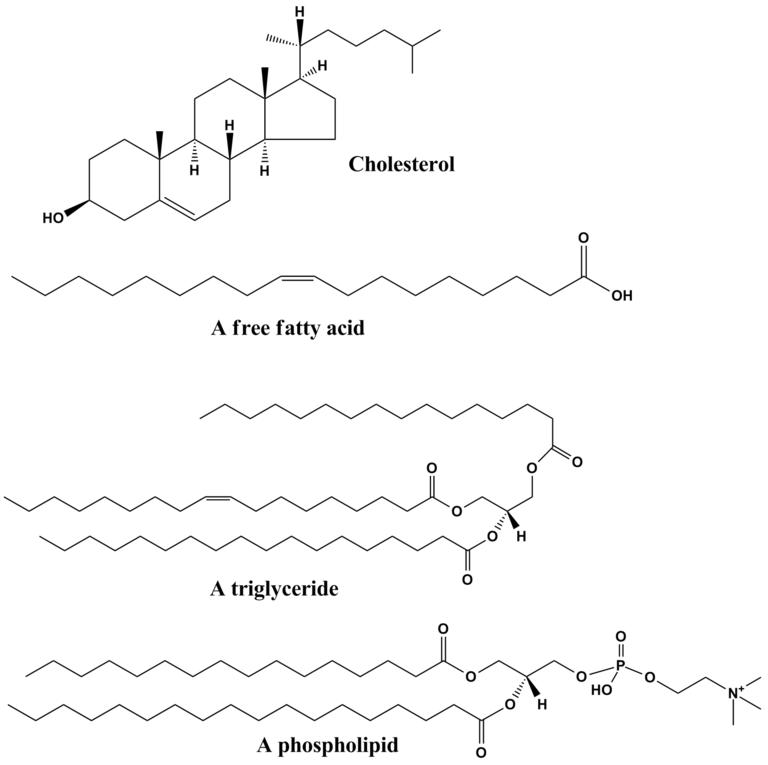
Lipids
Lipids are molecules that are hydrophobic (insoluble in water) because the non-polar covalent bonds linking carbon and hydrogen aren’t attracted to the polar bonds of water. The four major categories of lipids include fats, phospholipids, waxes and steroids.