| ||||
XX
Organic Chemistry:
What Is a Carbohydrate?
CLASS NOTES from Science Prof Online
You may recognize carbohydrates as source of energy (starch, glycogen), but they fulfill a wide range of roles, including the structural materials of plants (cellulose in plant cell walls) and of some animals (chitin of an insect’s exoskeleton). Carbohydrates can also be components of other molecules such as DNA, RNA, glycolipids, glycoproteins, and adenosine triphosphate (ATP).
Saccharides
The word saccharide is a synonym for carbohydrate and is generally preceded with a prefix indicating the size of the molecule (mono-, di-, tri- poly-).
Monosaccharides:
- single sugars (one molecule)
- simplest
- examples are glucose and fructose
Disaccharides:
- double sugars
- combination of two monosaccharides
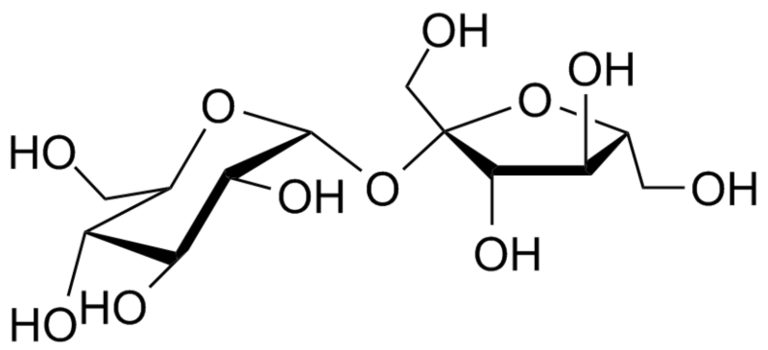
- sucrose = glucose + fructose
- lactose = glucose + galactose
Polysaccharides:
- polymers composed of several sugars
- can be same monomer (many of same monosaccharide) or mixture of monomers
- glycogen is the major stored carbohydrate in animals
- starch is storage polysaccharide of plants. It is a long chain of glucose molecules.
- chitin is a structural carb in some animals
- cellulose is the major structural carbohydrate in plants
Building and Breaking Down Sugars
Dehydration and hydrolysis are chemical reactions that make molecules bigger (dehydration) and smaller (hydrolysis).
Dehydration Reactions: Dehydration is when one molecule contributes a hydrogen (H) and the other a hydroxyl group (OH), therefore the removal of a water molecule (H2O) results in the joining of two smaller molecules. With respect to carbohydrates, dehydration reactions make bigger carbohydrate molecules from smaller sugars.
Hydrolysis Reactions: Hydrolysis is the reverse of dehydration and is when the addition of a water molecule breaks (lyses) a larger molecule into two smaller molecules. With respect to carbohydrate hydrolysis, the bonds on the larger carbohydrate are broken through the addition of water. One of the smaller molecules receives a hydrogen (H) and the other received a hydroxyl group (OH).
Sources and Resources
- Bauman, R. (2014) Microbiology with Diseases by Taxonomy, 4th ed., Pearson Benjamin Cummings.
- Park Talaro, K. (2008) Foundations in Microbiology. McGraw-Hill.
- Biomolecules: The Carbohydrates interactive animated lesson from Wisc-Online.com.
- Interactive Concepts in Biochemistry Interactive Animations.
- Fundamentals of Biochemistry Biochem Animations from Wiley.
Article Summary: Carbs, also known as saccharides, are organic molecules that are used as energy sources, structural molecules and as components of other biological molecules.
Mono-, Di - & Poly- Saccharide Sugars
You have free access to a large collection of materials used in a college-level introductory Cell Biology Course. The Virtual Cell Biology Classroom provides a wide range of free educational resources including Power Point Lectures, Study Guides, Review Questions and Practice Test Questions.
 | ||||||
SPO VIRTUAL CLASSROOMS
Organic molecules are large chemical compounds that contain carbon-hydrogen bonds and are found in living things. The major classes of organic molecules are carbohydrates, proteins,
Carbohydrates
The term carbohydrate is actually a descriptor of what these molecules are composed of. They are “carbon hydrates,” in a ratio of one carbon molecule to one water molecule (CH2O)n.
 | ||||||
SPO is a FREE science education website. Donations are key in helping us provide this resource with fewer ads.
Please help!
(This donation link uses PayPal on a secure connection.)
You have free access to a large collection of materials used in a college-level introductory microbiology course. The Virtual Microbiology Classroom provides a wide range of free educational resources including PowerPoint Lectures, Study Guides, Review Questions and Practice Test Questions.
Page last updated: 9/2014
Sucrose, also known as table sugar, is a common disaccharide. It is composed of two monosaccharides: D-glucose (left) and D-fructose (right).