| ||||
Atomic Theory & the Periodic Table
Review Questions
from Science Prof Online
Atomic Theory & Periodic Table Review Qus
3. Describe the subatomic parts of an atom, including any charge they have.
4. What is the difference between "atomic number", "atomic mass" & "mass number"?
5. What are isotopes. How does the "atomic mass" relate to isotopes?
6. What are valence electrons and why are they important? What is the maximum number of electrons that an atom can have in its valence shell?
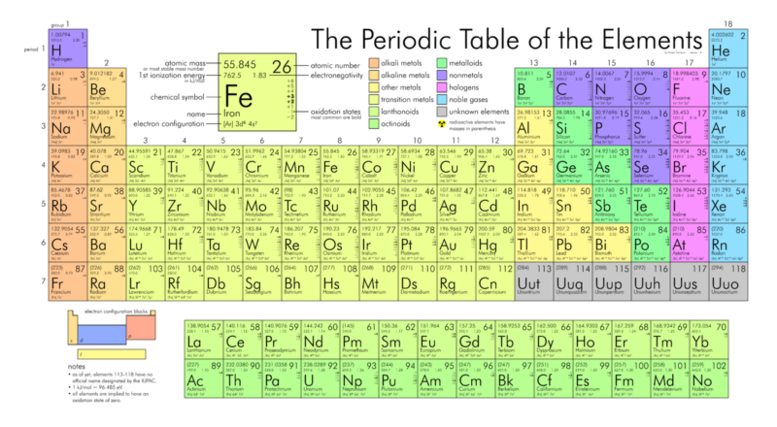
7. What is the difference between "groups" and "periods" on the periodic table?
Create a labeled electron shell diagram of the following elements, showing number of protons, neutrons and electrons in their proper orbitals.
8. Oxygen
9. Aluminum
10. Magnesium
Atomic Theory & the Periodic Table
Free multiple choice and true/false questions designed to help students practice and test their understanding of Atomic Theory & the Periodic Table.
 | ||||||
SPO VIRTUAL CLASSROOMS
Page last updated: 5/2016
1. Specifically, what is the atomic number of an element based on?
2. What is the relationship between an atom and an element? How and when do they differ?
These are review questions from the Virtual Cell Biology Classroom designed to help students better understand Inorganic Chemistry. They are based on materials that can be found on the Atomic Theory & Periodic Table Lecture Main Page.
Click here for a printable, detailed periodic table.
You have free access to a large collection of materials used in a college-level introductory Cell Biology Course. The Virtual Cell Biology Classroom provides a wide range of free educational resources including Power Point Lectures, Study Guides, Review Questions and Practice Test Questions.