| ||||
What Is a
Concentration Gradient?
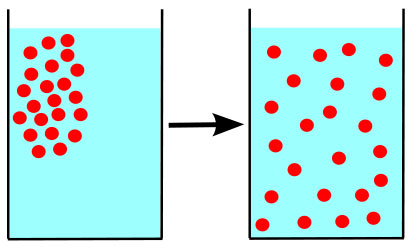
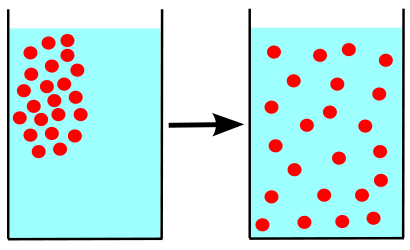
A difference in concentration of molecules in physical space is called a concentration gradient. An easy to understand example from everyday life is the application and subsequent fading of perfume or aftershave scent.
Article Summary: What is a concentration gradient and how does it provide energy for the movement of molecules? Here's a simple explanation.
What Is a Concentration Gradient?
Second hand smoke that you breathe in when near a smoker has traveled down a concentration gradient to you.
 | ||||||
SPO VIRTUAL CLASSROOMS
Everyday Examples of Concentration Gradient
The concentration of scent molecules is highest on areas of the skin that have had perfume or aftershave directly applied. Others can smell the scent because some of those molecules are always traveling away from the perfumed person, the source, out into the air—moving down the concentration gradient, from a high concentration to a lower concentration. Eventually the scent molecules are so widely dispersed that they can no longer be detected.
Page last updated:
9/2015
You have FREE access to a large collection of materials used in a college-level introductory biology course. The Virtual Biology Classroom provides a wide range of free educational resources including PowerPoint Lectures, Study Guides, Review Questions & Practice Test Questions.
The SPO website is best viewed in Google Chrome,
Microsoft Explorer or Apple Safari.
Molecules not only travel through air, but through other media as well. When a person puts creamer in his or her coffee, the cream molecules would eventually bounce around in the cup, moving down the concentration gradient until evenly distributed. However, most coffee drinkers do not wait for this to happen. They introduce additional energy by stirring the coffee and speeding up the process.
Concentration Gradients and the Plasma Membrane of Biological Cells
The plasma membrane controls traffic of materials moving into and out of biological cells. It is composed primarily of phospholipids and proteins, and is semi-permeable, allowing some materials to easily move across and barring the transport of others.
Think about the unpleasant scent of skunk stink, when a skunk has sprayed or been struck by a vehicle. As a person gets closer to the source of the skunk stink, the scent gets stronger, because the “stink molecules” are more highly concentrated closer to the source.